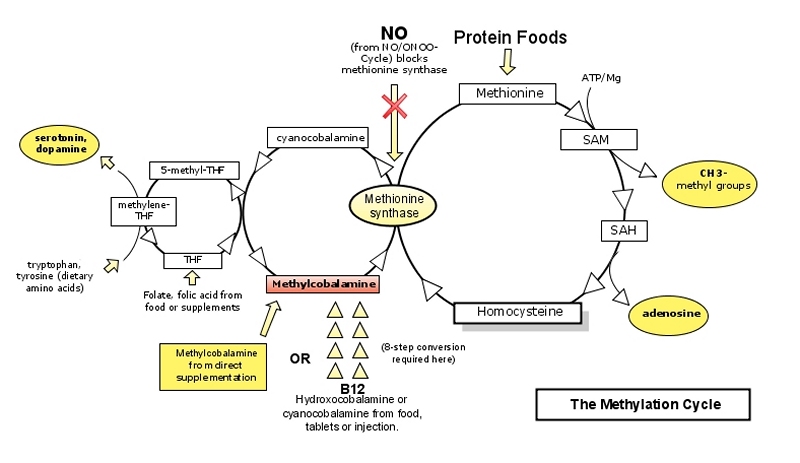
Figure 3: The Methylation Cycle
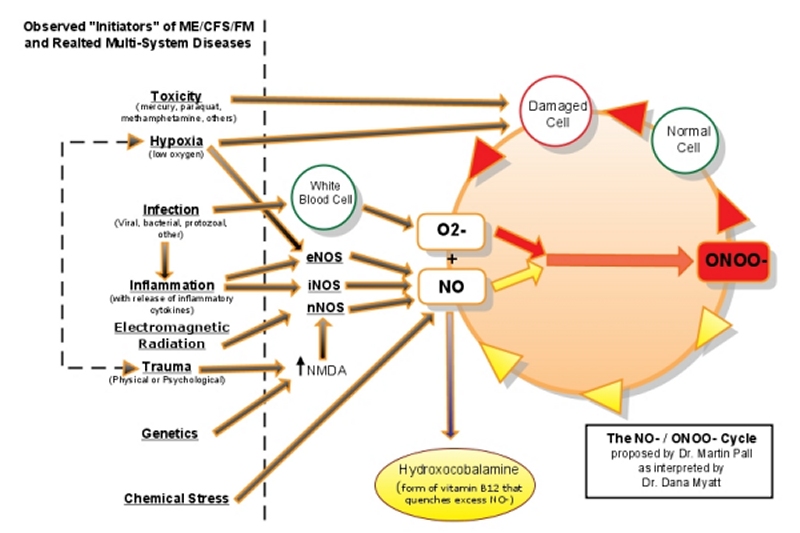
Note the overlap between the NO‾/ONOO‾ Cycle and the Methylation Cycle where excess NO‾ blocks methionine synthase, a critical enzyme in the methylation cycle. (106, 164-167)
The methylcobalamin form of vitamin B-12 is a required nutrient in the Methylation Cycle. If any one step in the methylation cycle fails, the entire cycle fails.
Vitamin B12: Which Form is Best?
What we know as Vitamin B-12 is actually a collection of four related but different cobalt-containing molecules. Each of these forms plays a distinct role in the body as follows:
Hydroxycobalamin is a unique form of B12 that quenches excess nitric oxide (NO‾), the precursor to peroxinitrite (ONOO‾).(147-149,172-176) Hydroxocobalamine (and methylcobalamine) are also more effective at treating neurological disorders than cyanocobalamine. (168)
Hydroxocobalamin participates in detoxification, especially cyanide detoxification. Cyanide levels are typically elevated in smokers, people who eat cyanide-containing food (like cassava) and those with certain metabolic defects. Excess cyanide in the tissues blocks conversion of cyanocobalamin to methylcobalamin or adenosylcobalamin. In such instances, hydroxocobalamin is the vitamin B-12 of choice. (169-171) Hydroxycobalamin is FDA- approved as a treatment for cyanide poisoning. (214
Methylcobalamin is considered by many researchers to be the most active form of vitamin B12. (177-179) It is the requisite form of vitamin B-12 in the Methylation Cycle. (179-186). Methylcobalamin protects cortical neurons against NMDA receptor-mediated glutamate cytotoxicity.(187-188) and promotes nerve cell regeneration. (189) Methylcobalamine is the only form of vitamin B-12 that participates in regulating circadian rhythms (sleep/wake cycles). It has been shown to improve sleep quality and refreshment from sleep, as well as increased feeling of well-being, concentration and alertness. (190).
Adenosylcobalamin (dibencozide), another highly active form of vitamin B12, is essential for energy metabolism (191) and is required for normal myelin sheath formation and nucleoprotein synthesis. Deficiencies are associated with nerve and spinal cord degeneration. (192-193)
Cyanocobalamin, the most common form of B12 found in nutritional supplements, is a synthetic form of B12 not found in nature. It has the lowest biological activity and must be converted in the liver to more biologically active forms. This conversion is inefficient and some people who may not benefit from cyanocobalamine due to lack of assimilation or conversion. (194-195) However, the cyano form of B12 is needed to balance hydroxycobalamin in performing its NO-quenching function and should therefore be included in hydroxocobalamine supplements. (176)
Who is Vitamin B12 Deficient and Why?
Research shows that a much larger segment of the general population is vitamin B12 deficient than previously thought. Recent studies indicate that up to 78% of seniors are deficient. (196-197)
Irritable bowel syndrome (IBS), seen in as many as 77% of CFS patients and 78% of FM patients (198-199) is a major cause of vitamin B12 deficiency. (200) This leads one to ponder the “which came first, the chicken or egg” nature of this: are ME/CFS patients B12 deficient because of IBS, or is IBS a result of cellular or neurological insult caused by B12 deficiency?
Other high-risk groups for B12 deficiency include those who use acid-blocking or neutralizing drugs (such as Prilosec, Prevacid, Nexium and others) (201-204), drugs which impair intestinal absorption (such as Metformin, Questron and Chloromycetin) (205), and people who have had gastric surgery. (206-207) Bacterial overgrowth of the small intestine, which occurs frequently in people with ME/CFS and low stomach acid, is a predisposing factor for B12 deficiency because the bacteria themselves use vitamin B12. (208-209)
The most recent and disturbing studies suggest that vitamin B12 deficiency is more prevalent in young adults than previously thought. (210-211). One study found that vitamin B12 deficiency was similar in three age groups (26-49 years, 50-64 years, and 65 years and older), but that early symptoms were simply less apparent in the young. This study also found that those who did not take a vitamin B12-containing supplement were twice as likely to be deficient as supplement users, regardless of age. (210)
Secondly, unlike other water-soluble vitamins, B12 is stored in the liver, kidneys and other tissues. Deficiencies of B12 often appear so slowly and subtly as to go unnoticed, and blood tests for vitamin B12 levels miss early deficiency states at least 50% of the time. (212-213)
Why Vitamin B12 MUST Be Obtained From Supplements
Medical science once believed that few people were vitamin B12 deficient. This false assumption may stem from the fact that vitamin B12 is produced in the body by a normal, healthy population of bowel bacteria.
Foods are not a significant source of vitamin B12. Meat, milk, eggs, fish, and shellfish contain the highest amount of B12 but only 50% of this is absorbable even in a healthy gut. (215) Vegetarian sources of vitamin B12, such as algae, are not bio-available and do not make significant contribution to dietary vitamin B12 levels. (216)
Further, absorption is hampered by low stomach acid, IBS, and bacterial overgrowth of the small intestine --- conditions which are common in ME/CFS sufferers. The US Institute of Medicine recommends that adults over 50 obtain their vitamin B12 from supplements. (14)
Oral vs. Injectable: Which is Best?
Although vitamin B12 has previously been given by injection, it is now accepted in conventional medicine that oral vitamin B12 is equally as effective as injection in treating pernicious anemia and other B12 deficient states. (214, 217-220).
According to The National Institutes of Health (NIH), oral vitamin B12 supplementation is extremely safe (221-222). It is also as effective as injections, (14,219-220) and inexpensive and more convenient compared to injection. (220)
All Roads Lead To B12:
Conclusions and Recommendations
The suffering from ME/CFS and other multi-system diseases is widespread and devastating. This affliction is beginning to receive more attention, perhaps because of the activism of those affected and the dedication of ME/CFS researchers and clinicians. Current research is providing us with new insights into the underlying mechanisms of this complicated illness.
The Nitric Oxide / Peroxynitrite model (NO‾/ONOO‾) and The Methylation Cycle have emerged as two likely contributory mechanisms to ME/CFS and other multi-system diseases including Fibromyalgia (FM), Lyme Disease, Multiple Chemical Sensitivities (MCS), PTSD and Gulf War Syndrome. Deficiencies of either of two forms of vitamin B12 --- hydroxocobalamin and/or methylcobalamin --- play a significant role in these biochemical processes.
Since Vitamin B12 (especially the hydroxocobalamin and methycobalamin forms) offer such potential benefits for ME/CFS and other multi-system disease sufferers --- without known risks --- it seems reasonable to suggest that anyone suffering with ME/CFS or other multi-system illness should consider taking a supplement containing these two important forms of vitamin B12.
Furthermore, because of the balancing effect that cyanocobalamin has on hydroxycobalamin (176) and the protective and regenerative effect that adenosylcobalamin exerts on the myelin sheath of nerves (192-193), these forms should also be considered as an important part of any complete vitamin B12 supplement.
References:
1.) http://www.ncf-net.org/conference/CheneyLecture.htm
2.) http://www.ncf-net.org/forum/lapp97.htm
3.)http://www.immunesupport.com/Library/showarticle.cfm/ID/4907/Hea
lthWatch/HealthWatch-Treatment-Guide-2003
4.) http://www.immunesupport.com/library/showarticle.cfm/id/4337 -
5.)http://www.immunesupport.com/library/showarticle.cfm/ID/4351
6.)Explaining 'Unexplained Illnesses': Disease Paradigm for Chronic
Fatigue Syndrome, Multiple Chemical Sensitivity, Fibromyalgia,
Post-Traumatic Stress Disorder, and Gulf War Syndrome; Martin Pall:
Harrington Park Press; 1 edition (May 15, 2007) Page 106.
7.Ellis FR, Nasser S. A pilot study of vitamin B12 in the treatment
of tiredness.Br J Nutr 1973;30:277–83.
8.Lapp CW, Cheney PR. The rationale for using high-dose cobalamin
(vitamin B12). CFIDS Chronicle
Physicians’ Forum, 1993;Fall:19–20.
9.) Herbert V. Vitamin B12 in Present Knowledge in Nutrition. 17th
ed. Washington, D.C.:
International Life Sciences Institute Press, 1996.
10.) Combs G. Vitamin B12 in The Vitamins. New York: Academic
Press, Inc, 1992.
11.) Herbert V and Das K. Vitamin B12 in Modern Nutrition in
health and disease. 8th ed. Baltimore: Williams & Wilkins, 1994.
12.) Zittoun J and Zittoun R. Modern clinical testing strategies
in cobalamin and folate deficiency. Sem Hematol 1999;36:35-46.
13.) Healton EB, Savage DG, Brust JC, Garrett TF, Lindenbaum J.
Neurological aspects of cobalamin deficiency. Medicine
1991;70:229-244.
14.) Institute of Medicine. Food and Nutrition Board. Dietary
Reference Intakes: Thiamin, riboflavin, niacin, vitamin B6,
folate, vitamin B12, pantothenic acid, biotin, and choline.
National Academy Press. Washington, DC, 1998.
15.) Bottiglieri T. Folate, vitamin B12, and neuropsychiatric
disorders. Nutr Rev 1996;54:382-90.
16.) Roze E, Gervais D, Demeret S, Ogier de Baulny H, Zittoun J,
Benoist JF, Said G, Pierrot-Deseilligny C, Bolgert
F.Neuropsychiatric disturbances in presumed late-onset cobalamin C
disease.Arch Neurol. 2003 Oct;60(10):1457-62.
17.) van Goor L, Woiski MD, Lagaay AM, Meinders AE, Tak PP.Review:
cobalamin deficiency and mental impairment in elderly people. Age
Ageing. 1995 Nov;24(6):536-42.
18.) Martin DC, Francis J, Protetch J, Huff FJ. Time dependency of
cognitive recovery with cobalamin replacement: report of a pilot
study. J Am Geriatr Soc. 1992 Feb;40(2):168-72.
19) Robertson JS, Hsia YE, Scully KJ.Defective leukocyte metabolism
in human cobalamin defieciency: impaired propionate oxidation and
serine biosynthesis reversible by cyanocobalamin therapy.J Lab Clin
Med. 1976 Jan;87(1):89-97.
20) Tamura J, Kubota K, Murakami H, Sawamura M, Matsushima T,
Tamura T, Saitoh T, Kurabayshi H, Naruse T. Immunomodulation by
vitamin B12: augmentation of CD8+ T lymphocytes and natural killer
(NK) cell activity in vitamin B12-deficient patients by methyl-B12
treatment. Clin Exp Immunol. 1999 Apr;116(1):28-32.
21) Fenech MF, Dreosti IE, Rinaldi JR.Folate, vitamin B12,
homocysteine status and chromosome damage rate in lymphocytes of
older men. Carcinogenesis. 1997 Jul;18(7):1329-36
22.) Third Report of the National Cholesterol Education Program
Expert Panel on Detection, Evaluation, and Treatment of High Blood
Cholesterol in Adults (Adult Treatment Panel III). National
Cholesterol Education Program, NationalHeart, Lung, and Blood
Institute, National Institues of Health, September 2002. NIH
Publication No. 02-5215.
23) Selhub J, Jacques PF, Bostom AG, D'Agostino RB, Wilson PW,
Belanger AJ, O'Leary DH, Wolf PA, Scaefer EJ, Rosenberg IH.
Association between plasma homocysteine concentrations and
extracranial carotid-artery stenosis. N Engl J Med
1995;332:286-91.
24) Rimm EB, Willett WC, Hu FB, Sampson L, Colditz G A, Manson J
E, Hennekens C, Stampfer M J. Folate and vitamin B6 from diet and
supplements in relation to risk of coronary heart disease among
women. J Am Med Assoc 1998;279:359-64.
25) Refsum H, Ueland PM, Nygard O, Vollset SE. Homocysteine and
cardiovascular disease. Annu Rev Med 1998;49:31-62
26) Boers GH. Hyperhomocysteinemia: A newly recognized risk factor
for vascular disease. Neth J Med 1994;45:34-41.
27) Selhub J, Jacques PF, Wilson PF, Rush D, Rosenberg IH. Vitamin
status and intake as primary determinants of homocysteinemia in an
elderly population. J Am Med Assoc 1993;270:2693-8.
28) Malinow MR. Plasma homocyst(e)ine and arterial occlusive
diseases: A mini-review. Clin Chem 1995;41:173-6
29) Flynn MA, Herbert V, Nolph GB, Krause G. Atherogenesis and the
homocysteine-folate-cobalamin triad: do we need standardized
analyses? J Am Coll Nutr 1997;16:258-67.
30) Fortin LJ, Genest J, Jr. Measurement of homocyst(e)ine in the
prediction of arteriosclerosis. Clin Biochem 1995;28:155-62
31.) Siri PW, Verhoef P, Kok FJ. Vitamins B6, B12, and folate:
Association with plasma total homocysteine and risk of coronary
atherosclerosis. J Am Coll Nutr 1998;17:435-41.
32.) Ubbink JB, van der Merwe A, Delport R, Allen R H, Stabler S
P, Riezler R, Vermaak WJ. The effect of a subnormal vitamin B6
status on homocysteine metabolism. J Clin Invest 1996;98:177-84.
33) Bronstrup A, Hages M, Prinz-Langenohl R, Pietrzik K. Effects
of folic acid and combinations of folic acid and vitamin B12 on
plasma homocysteine concentrations in healthy, young women. Am J
Clin Nutr 1998;68:1104-10.
34) Remacha AF, Souto JC, Rámila E, Perea G, Sarda MP, Fontcuberta
J.Enhanced risk of thrombotic disease in patients with acquired
vitamin B12 and/or folate deficiency: role of
hyperhomocysteinemia.Ann Hematol. 2002 Nov;81(11):616-21. Epub 2002
Nov 9.
35.) Beers, M.H., Berkow, R. et al. The Merck Manual of Diagnosis
and Therapy, Seventeenth Edition, 1999 Merck and Co., Chapter 127
page 867.
36.) Monsen ALB and Ueland PM. Homocysteine and methylmalonic acid
in diagnosis and risk assessment from infancy to adolescent.
American Journal of Clinical Nutrition 2003; 78:7-21.
37.) Carmel R. Megaloblastic anemias. Curr Opin Hematol
1994;1:107-12.
38.) Soderlund A, Malterud K. Why did I get chronic fatigue
syndrome? A qualitative interview study of causal attributions in
women patients. Scand J Prim Health Care. 2005 Dec;23(4):242-7.
39.) Kerr JR, Tyrrell DA. Cytokines in parvovirus B19 infection as
an aid to understanding chronic fatigue syndrome. Curr Pain
Headache Rep. 2003 Oct;7(5):333-41.
40.) Ferguson E, Cassaday HJ. Theoretical accounts of Gulf War
Syndrome: from environmental toxins to psychoneuroimmunology and
neurodegeneration. Behav Neurol. 2001-2002;13(3-4):133-47.
41.) Peckerman A, LaManca JJ, Smith SL, Taylor A, Tiersky L, Pollet
C, Korn LR, Hurwitz BE, Ottenweller JE, Natelson BH. Cardiovascular
stress responses and their relation to symptoms in Gulf War
veterans with fatiguing illness. Psychosom Med. 2000
Jul-Aug;62(4):509-16.
42.) Skowera A, Hotopf M, Sawicka E, Varela-Calvino R, Unwin C,
Nikolaou V, Hull L, Ismail K, David AS, Wessely SC, Peakman
M.Cellular immune activation in Gulf War veterans. J Clin Immunol.
2004 Jan;24(1):66-73.
43.) Patarca R. Cytokines and chronic fatigue syndrome. Ann N Y
Acad Sci. 2001 Mar;933:185-200.
44.) Lucas HJ, Brauch CM, Settas L, Theoharides TC.
Fibromyalgia--new concepts of pathogenesis and treatment. Int J
Immunopathol Pharmacol. 2006 Jan-Mar;19(1):5-10.
45.) Mease P. Fibromyalgia syndrome: review of clinical
presentation, pathogenesis, outcome measures, and treatment. J
Rheumatol Suppl. 2005 Aug;75:6-21.
46.) Fiedler N, Kipen HM, DeLuca J, Kelly-McNeil K, Natelson B. A
controlled comparison of multiple chemical sensitivities and
chronic fatigue syndrome. Psychosom Med. 1996 Jan-Feb;58(1):38-49.
47.) Gaudino EA, Coyle PK, Krupp LB. Post-Lyme syndrome and chronic
fatigue syndrome. Neuropsychiatric similarities and differences.
Arch Neurol. 1997 Nov;54(11):1372-6.
48.) Jason LA, Taylor RR, Kennedy CL. Chronic fatigue syndrome,
fibromyalgia, and multiple chemical sensitivities in a
community-based sample of persons with chronic fatigue
syndrome-like symptoms. Psychosom Med. 2000 Sep-Oct;62(5):655-63.
49.) Kang HK, Natelson BH, Mahan CM, Lee KY, Murphy FM.
Post-traumatic stress disorder and chronic fatigue syndrome-like
illness among Gulf War veterans: a population-based survey of
30,000 veterans. Am J Epidemiol. 2003 Jan 15;157(2):141-8.
50.) Aaron LA, Burke MM, Buchwald D. Overlapping conditions among
patients with chronic fatigue syndrome, fibromyalgia, and
temporomandibular disorder.Arch Intern Med. 2000 Jan
24;160(2):221-7.
51.) Paul M. Explaining 'Unexplained Illnesses': Disease Paradigm
for Chronic Fatigue Syndrome, Multiple Chemical Sensitivity,
Fibromyalgia, Post-Traumatic Stress Disorder, and Gulf War
Syndrome. The Hawthorne Press Inc., 2007.
52.) McCann SM, Licinio J, Wong ML, Yu WH, Karanth S, Rettorri V.
The nitric oxide hypothesis of aging. Exp Gerontol. 1998
Nov-Dec;33(7-8):813-26.
53.) Jeong JH, Kum C, Choi HJ, Park ES, Sohn UD. Extremely low
frequency magnetic field induces hyperalgesia in mice modulated by
nitric oxide synthesis. Life Sci. 2006 Feb 23;78(13):1407-12. Epub
2006 Feb 7.
54.) Ródenas J, Mitjavila MT, Carbonell T. Simultaneous generation
of nitric oxide and superoxide by inflammatory cells in rats. Free
Radic Biol Med. 1995 May;18(5):869-75.
55.) Zingarelli B, Scott GS, Hake P, Salzman AL, Szabo C. Effects
of nicaraven on nitric oxide-related pathways and in shock and
inflammation. Shock. 2000 Feb;13(2):126-34.
56.) Daghigh F, Borghaei RC, Thornton RD, Bee JH. Human gingival
fibroblasts produce nitric oxide in response to proinflammatory
cytokines. J Periodontol. 2002 Apr;73(4):392-400.
57.) Guix FX, Uribesalgo I, Coma M, Muñoz FJ.The physiology and
pathophysiology of nitric oxide in the brain. Prog Neurobiol. 2005
Jun;76(2):126-52.
58.) Molina JA, Jiménez-Jiménez FJ, Ortí-Pareja M, Navarro JA.The
role of nitric oxide in neurodegeneration. Potential for
pharmacological intervention. Drugs Aging. 1998 Apr;12(4):251-9.
59.) Larson AA, Giovengo SL, Russell IJ, Michalek JE. Changes in
the concentrations of amino acids in the cerebrospinal fluid that
correlate with pain in patients with fibromyalgia: implications for
nitric oxide pathways. Pain. 2000 Aug;87(2):201-11.
60.) Zhan G, Fenik P, Pratico D, Veasey SC.Inducible nitric oxide
synthase in long-term intermittent hypoxia: hypersomnolence and
brain injury. Am J Respir Crit Care Med. 2005 Jun
15;171(12):1414-20. Epub 2005 Mar 4.
61.) Hesse AK, Dörger M, Kupatt C, Krombach F.Proinflammatory role
of inducible nitric oxide synthase in acute hyperoxic lung
injury.Respir Res. 2004 Sep 15;5:11.
62.) Lehtonen H, Oksa P, Lehtimäki L, Sepponen A, Nieminen R,
Kankaanranta H, Saarelainen S, Järvenpää R, Uitti J, Moilanen
EIncreased alveolar nitric oxide concentration and high levels of
leukotriene B(4) and 8-isoprostane in exhaled breath condensate in
patients with asbestosis. Thorax. 2007 Jul;62(7):602-7. Epub 2007
Jan 24.
63.) Imam SZ, Islam F, Itzhak Y, Slikker W Jr, Ali SF. Prevention
of dopaminergic neurotoxicity by targeting nitric oxide and
peroxynitrite: implications for the prevention of
methamphetamine-induced neurotoxic damage. Ann N Y Acad Sci. 2000
Sep;914:157-71.
64.) Sviriaeva IV, Ruuge EK, Shumaev KB.Formation of superoxide
radicals in isolated cardiac mitochondria: effect of adriamycin.
Biofizika. 2007 Nov-Dec;52(6):1054-9.[PubMed abstract; article in
Russian].
65.) Doroshow JH. Effect of anthracycline antibiotics on oxygen
radical formation in rat heart. Cancer Res. 1983 Feb;43(2):460-72.
66.) Yurkov IS, Kruglov AG, Evtodienko YV, Yaguzhinsky LS.
Mechanism of superoxide anion generation in intact mitochondria in
the presence of lucigenin and cyanide. Biochemistry (Mosc). 2003
Dec;68(12):1349-59.
67.) Pacher, P.; Beckman, J. S.; Liaudet, L.; “Nitric Oxide and
Peroxynitrite: in Health and disease” Physiological Reviews 2007,
volume 87(1), page 315-424.
68.) Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite
oxidation of sulfhydryls. The cytotoxic potential of superoxide and
nitric oxide. J Biol Chem. 1991 Mar 5;266(7):4244-50. 69.) Pryor
WA, Squadrito GL.The chemistry of peroxynitrite: a product from the
reaction of nitric oxide with superoxide. Am J Physiol. 1995
May;268(5 Pt 1):L699-722.
70.) Beckman, J.S., Koppenol, W.H. Nitric oxide, superoxide, and
peroxynitrite: The good, the bad, and the ugly. Am J Physiol 271
C1424-C1437 (1996).
71.) Koppenol, W.H., Moreno, J.J., Pryor, W.A., et al.
Peroxynitrite, a cloaked oxidant formed by nitric oxide and
superoxide. Chem Res Toxicol 5 834-842 (1992).
72.) Squadrito GL, Pryor WA.Oxidative chemistry of nitric oxide:
the roles of superoxide, peroxynitrite, and carbon dioxide. Free
Radic Biol Med. 1998 Sep;25(4-5):392-403.
73.) Oku H, Fukuhara M, Komori A, Okuno T, Sugiyama T, Ikeda
T.Endothelin-1 (ET-1) causes death of retinal neurons through
activation of nitric oxide synthase (NOS) and production of
superoxide anion. Exp Eye Res. 2008 Jan;86(1):118-30. Epub 2007 Oct
9.
74.) Pérez-De La Cruz V, González-Cortés C, Galván-Arzate S,
Medina-Campos ON, Pérez-Severiano F, Ali SF, Pedraza-Chaverrí J,
Santamaría A. Excitotoxic brain damage involves early peroxynitrite
formation in a model of Huntington's disease in rats: protective
role of iron porphyrinate 5,10,15,20-tetrakis
(4-sulfonatophenyl)porphyrinate iron (III). Neuroscience.
2005;135(2):463-74.
75.) Shibuki H, Katai N, Yodoi J, Uchida K, Yoshimura N. Lipid
peroxidation and peroxynitrite in retinal ischemia-reperfusion
injury. Invest Ophthalmol Vis Sci. 2000 Oct;41(11):3607-14. 76.)
Kawano T, Kunz A, Abe T, Girouard H, Anrather J, Zhou P, Iadecola
C. iNOS-derived NO and nox2-derived superoxide confer tolerance to
excitotoxic brain injury through peroxynitrite. J Cereb Blood Flow
Metab. 2007 Aug;27(8):1453-62. Epub 2007 Feb 7. 77.) Zingarelli B,
Scott GS, Hake P, Salzman AL, Szabo C. Effects of nicaraven on
nitric oxide-related pathways and in shock and inflammation. Shock.
2000 Feb;13(2):126-34.
78.) Zingarelli B, Day BJ, Crapo JD, Salzman AL, Szabó C. The
potential role of peroxynitrite in the vascular contractile and
cellular energetic failure in endotoxic shock. Br J Pharmacol. 1997
Jan;120(2):259-67.
79.) Pryor, W.A., Squadrito, G.L. The chemistry of peroxynitrite: A
product from the reaction of nitric oxide with superoxide. Am J
Physiol 268 L699-L722 (1995).
80.) Aquaro S, Muscoli C, Ranazzi A, Pollicita M, Granato T,
Masuelli L, Modesti A, Perno CF, Mollace V. The contribution of
peroxynitrite generation in HIV replication in human primary
macrophages. Retrovirology. 2007 Oct 21;4:76.
81.) Mendoza MG, Castillo-Henkel C, Medina-Santillan R, Jarillo
Luna RA, Robles HV, Romo E, Rios A, Escalante B. Kidney damage
after renal ablation is worsened in endothelial nitric oxide
synthase -/- mice and improved by combined administration of
L-arginine and antioxidants. Nephrology (Carlton). 2008
Jun;13(3):218-27.
82.) Piao XL, Cho EJ, Jang MH. Cytoprotective effect of baicalein
against peroxynitrite-induced toxicity in LLC-PK(1) cells.Food Chem
Toxicol. 2008 May;46(5):1576-81. Epub 2007 Dec 31.
83.) Kimoto K, Aoki T, Shibata Y, Kamisuki S, Sugawara F, Kuramochi
K, Nakazaki A, Kobayashi S, Kuroiwa K, Watanabe N, Arai T.
Structure-activity relationships of neoechinulin A analogues with
cytoprotection against peroxynitrite-induced PC12 cell death. J
Antibiot (Tokyo). 2007 Oct;60(10):614-21.
84.) Jack C, Antel J, Brück W, Kuhlmann T. Contrasting potential of
nitric oxide and peroxynitrite to mediate oligodendrocyte injury in
multiple sclerosis. Glia. 2007 Jul;55(9):926-34.
85.) Martinez-Palma L, Pehar M, Cassina P, Peluffo H, Castellanos
R, Anesetti G, Beckman JS, Barbeito L. Involvement of nitric oxide
on kainate-induced toxicity in oligodendrocyte precursors.Neurotox
Res. 2003;5(6):399-406.
86.) Ali TK, Matragoon S, Pillai BA, Liou GI, El-Remessy AB.
Peroxynitrite Mediates Retinal Neurodegeneration by Inhibiting NGF
Survival Signal in Experimental and Human Diabetes. Diabetes. 2008
Feb 19 [Epub ahead of print].
87.) Ho SC, Tsai TH, Tsai PJ, Lin CC. Protective capacities of
certain spices against peroxynitrite-mediated biomolecular damage.
Food Chem Toxicol. 2008 Mar;46(3):920-8. Epub 2007 Oct 30.
88.) Pehar M, Vargas MR, Robinson KM, Cassina P, England P, Beckman
JS, Alzari PM, Barbeito L. Peroxynitrite transforms nerve growth
factor into an apoptotic factor for motor neurons. Free Radic Biol
Med. 2006 Dec 1;41(11):1632-44. Epub 2006 Aug 15.
89.) Jonnala RR, Buccafusco JJ.Inhibition of nerve growth factor
signaling by peroxynitrite. J Neurosci Res. 2001 Jan
1;63(1):27-34.
90.) Pessayre D. Role of mitochondria in non-alcoholic fatty liver
disease. J Gastroenterol Hepatol. 2007 Jun;22 Suppl 1:S20-7.
91.) Cejková J, Ardan T, Simonová Z, Cejka C, Malec J, Jirsová K,
Filipec M, Dotrelová D, Brunová B. Nitric oxide synthase induction
and cytotoxic nitrogen-related oxidant formation in conjunctival
epithelium of dry eye (Sjögren's syndrome).Nitric Oxide. 2007
Aug;17(1):10-7. Epub 2007 May 22.
92.) Szabó C, Day BJ, Salzman AL. Evaluation of the relative
contribution of nitric oxide and peroxynitrite to the suppression
of mitochondrial respiration in immunostimulated macrophages using
a manganese mesoporphyrin superoxide dismutase mimetic and
peroxynitrite scavenger. FEBS Lett. 1996 Feb 26;381(1-2):82-6.
93.) Chen Y, Gibson SB. Is mitochondrial generation of reactive
oxygen species a trigger for autophagy? Autophagy. 2008
Mar-Apr;4(2):246-8. Epub 2007 Dec 14.
94.) Szabó C, Zingarelli B, O'Connor M, Salzman AL.DNA strand
breakage, activation of poly (ADP-ribose) synthetase, and cellular
energy depletion are involved in the cytotoxicity of macrophages
and smooth muscle cells exposed to peroxynitrite. Proc Natl Acad
Sci U S A. 1996 Mar 5;93(5):1753-8.
94.) Goldstein S, Merényi G.The chemistry of peroxynitrite:
implications for biological activity. Methods Enzymol.
2008;436C:49-61.
95.) Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate
complex and uncoupling of endothelial nitric oxide synthase by
peroxynitrite. J Clin Invest. 2002 Mar;109(6):817-26.]
96.) Szabó C, O'Connor M, Salzman AL.Endogenously produced
peroxynitrite induces the oxidation of mitochondrial and nuclear
proteins in immunostimulated macrophages. FEBS Lett. 1997 Jun
9;409(2):147-50.
97.) Cuzzocrea S. Role of nitric oxide and reactive oxygen species
in arthritis. Curr Pharm Des. 2006;12(27):3551-70. 98.) Davies CM,
Guilak F, Weinberg JB, Fermor B. Reactive nitrogen and oxygen
species in interleukin-1-mediated DNA damage associated with
osteoarthritis. Osteoarthritis Cartilage. 2007 Oct 16 [Epub ahead
of print]
99.) Rui Hai Liu and Joseph H. Hotchkiss. Potential genotoxicity of
chronically elevated nitric oxide: A review. Mutation
Research/Reviews in Genetic Toxicology Volume 339, Issue 2, June
1995, Pages 73-89.
100.) Zhang L, Dawson VL, Dawson TM.Role of nitric oxide in
Parkinson's disease. Pharmacol Ther. 2006 Jan;109(1-2):33-41. Epub
2005 Jul 7. n
101.) Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield
DA, Stella AM.Nitric oxide in the central nervous system:
neuroprotection versus neurotoxicity. Nat Rev Neurosci. 2007
Oct;8(10):766-75.
102.) Zhan G, Fenik P, Pratico D, Veasey SC.Inducible nitric oxide
synthase in long-term intermittent hypoxia: hypersomnolence and
brain injury. Am J Respir Crit Care Med. 2005 Jun
15;171(12):1414-20. Epub 2005 Mar 4.
103.) Dawson VL, Dawson TM. Nitric oxide neurotoxicity.J Chem
Neuroanat. 1996 Jun;10(3-4):179-90.
104.) McCann SM. The nitric oxide hypothesis of brain aging. Exp
Gerontol. 1997 Jul-Oct;32(4-5):431-40.
105.) Petrosyan M, Perraki E, Simoes D, Koutsourelakis I, Vagiakis
E, Roussos C, Gratziou C. Exhaled breath markers in patients with
obstructive sleep apnoea. Sleep Breath. 2007 Dec 11 [Epub ahead of
print]. S
106.) Danishpajooh IO, Gudi T, Chen Y, Kharitonov VG, Sharma VS,
Boss GR. Nitric oxide inhibits methionine synthase activity in vivo
and disrupts carbon flow through the folate pathway. J Biol Chem.
2001 Jul 20;276(29):27296-303. Epub 2001 May 22.
107.) Campos AC, Molognoni F, Melo FH, Galdieri LC, Carneiro CR,
D'Almeida V, Correa M, Jasiulionis MG. Oxidative stress modulates
DNA methylation during melanocyte anchorage blockade associated
with malignant transformation. Neoplasia. 2007 Dec;9(12):1111-21.
108.) Brown GC. Nitric oxide and mitochondria.Front Biosci. 2007
Jan 1;12:1024-33.
109.) Jacobson J, Duchen MR, Hothersall J, Clark JB, Heales
SJ.Induction of mitochondrial oxidative stress in astrocytes by
nitric oxide precedes disruption of energy metabolism.J Neurochem.
2005 Oct;95(2):388-95. Epub 2005 Aug 16.
110.) Borutaite V, Moncada S, Brown GC.Nitric oxide from inducible
nitric oxide synthase sensitizes the inflamed aorta to hypoxic
damage via respiratory inhibition. Shock. 2005 Apr;23(4):319-23.
111.) Bal-Price A, Brown GC.Inflammatory neurodegeneration mediated
by nitric oxide from activated glia-inhibiting neuronal
respiration, causing glutamate release and excitotoxicity. J
Neurosci. 2001 Sep 1;21(17):6480-91.
112.) Brown GC, Borutaite V. Nitric oxide, mitochondria, and cell
death.IUBMB Life. 2001 Sep-Nov;52(3-5):189-95.
113.) Mander P, Borutaite V, Moncada S, Brown GC.Nitric oxide from
inflammatory-activated glia synergizes with hypoxia to induce
neuronal death. J Neurosci Res. 2005 Jan 1-15;79(1-2):208-15.
114.) Brown GC, Bal-Price A.Inflammatory neurodegeneration mediated
by nitric oxide, glutamate, and mitochondria. Mol Neurobiol. 2003
Jun;27(3):325-55.
115.) Packer MA, Murphy MP. Peroxynitrite formed by simultaneous
nitric oxide and superoxide generation causes
cyclosporin-A-sensitive mitochondrial calcium efflux and
depolarisation. Eur J Biochem. 1995 Nov 15;234(1):231-9.
116.) Jekabsone A, Neher JJ, Borutaite V, Brown GC. Nitric oxide
from neuronal nitric oxide synthase sensitises neurons to
hypoxia-induced death via competitive inhibition of cytochrome
oxidase.J Neurochem. 2007 Oct;103(1):346-56. Epub 2007 Jul 10.
117.) Packer MA, Porteous CM, Murphy MP.Superoxide production by
mitochondria in the presence of nitric oxide forms peroxynitrite.
Biochem Mol Biol Int. 1996 Oct;40(3):527-34.
118.) Inoue S, Kawanishi S. Oxidative DNA damage induced by
simultaneous generation of nitric oxide and superoxide. FEBS Lett.
1995 Aug 28;371(1):86-8.
119.) Szabó C.The pathophysiological role of peroxynitrite in
shock, inflamma
120.) Szabó C, Billiar TR. Novel roles of nitric oxide in
hemorrhagic shock.Shock. 1999 Jul;12(1):1-9.
121.) Maeda H, Akaike T.Nitric oxide and oxygen radicals in
infection, inflammation, and cancer. Biochemistry (Mosc). 1998
Jul;63(7):854-65.
122.) Akaike T.Role of free radicals in viral pathogenesis and
mutation. Rev Med Virol. 2001 Mar-Apr;11(2):87-101.
123.) Ebadi M, Sharma SK. Peroxynitrite and mitochondrial
dysfunction in the pathogenesis of Parkinson's disease. Antioxid
Redox Signal. 2003 Jun;5(3):319-35.
124.) Zaki MH, Akuta T, Akaike T.Nitric oxide-induced nitrative
stress involved in microbial pathogenesis. J Pharmacol Sci. 2005
Jun;98(2):117-29. Epub 2005 Jun 4.
125.) Akaike T, Suga M, Maeda H. Free radicals in viral
pathogenesis: molecular mechanisms involving superoxide and NO.Proc
Soc Exp Biol Med. 1998 Jan;217(1):64-73.
126.) Akaike T, Maeda H.Nitric oxide and virus
infection.Immunology. 2000 Nov;101(3):300-8.
127.) Akaike T, Fujii S, Kato A, Yoshitake J, Miyamoto Y, Sawa T,
Okamoto S, Suga M, Asakawa M, Nagai Y, Maeda H.Viral mutation
accelerated by nitric oxide production during infection in vivo.
FASEB J. 2000 Jul;14(10):1447-54.
128.) Fakhrzadeh L, Laskin JD, Laskin DL.Deficiency in inducible
nitric oxide synthase protects mice from ozone-induced lung
inflammation and tissue injury.Am J Respir Cell Mol Biol. 2002
Apr;26(4):413-9.
129.) Weinberger B, Fakhrzadeh L, Heck DE, Laskin JD, Gardner CR,
Laskin DL. Inhaled nitric oxide primes lung macrophages to produce
reactive oxygen and nitrogen intermediates. Am J Respir Crit Care
Med. 1998 Sep;158(3):931-8.
130.) Elsasser TH, Caperna TJ, Li CJ, Kahl S, Sartin JL. Critical
control points in the impact of proinflammatory immune response on
growth and metabolism. J Anim Sci. 2008 Mar 14 [Epub ahead of
print].
131.) FR Laurindo, PL da Luz, L Uint, TF Rocha, RG Jaeger and EA
Lopes. Evidence for superoxide radical-dependent coronary vasospasm
after angioplasty in intact dogs. Circulation, Vol 83, 1705-1715,
Copyright © 1991 by American Heart Association.
132.) Robert P. Jankov, Crystal Kantores, Jingyi Pan, Jaques Belik.
Contribution of xanthine oxidase-derived superoxide to chronic
hypoxic pulmonary hypertension in neonatal rats. Am J Physiol Lung
Cell Mol Physiol 294: L233-L245, 2008.
133.) Ricci C, Pastukh V, Leonard J, Turrens J, Wilson G, Schaffer
D, Schaffer SW. Mitochondrial DNA damage triggers
mitochondrial-superoxide generation and apoptosis.Am J Physiol Cell
Physiol. 2008 Feb;294(2):C413-22. Epub 2007 Dec 12.O2 134.)
Cuzzocrea S, Mazzon E, Dugo L, Di Paola R, Caputi AP, Salvemini
D.Superoxide: a key player in hypertension. FASEB J. 2004
Jan;18(1):94-101.
135.) Tomasz J Guzik, Shafi Mussa, Daniela Gastaldi, Jerzy
Sadowski, Chandi Ratnatunga, Ravi Pillai, and Keith M Channon.
Mechanisms of increased vascular superoxide production in human
diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric
oxide synthase. Circulation, 105(14):1656-62.
136.) Senthil Kumar Venugopal, Sridevi Devaraj, Teddy Yang, and
Ishwarlal Jialal. alpha Tocopherol Decreases Superoxide Anion
Release in Human Monocytes Under Hyperglycemic Conditions Via
Inhibition of Protein Kinase C. Diabetes 51:3049-3054, 2002.
137.) Alejandro MS Mayer, Mary L Hall, Sean M Lynch, Sarath P
Gunasekera, Susan H Sennett, Shirley A Pomponi. Differential
modulation of microglia superoxide anion and thromboxane B2
generation by the marine manzamines. BMC Pharmacology 2005,
5:6doi:10.1186/1471-2210-5-6.
138.) Zalba G, Beaumont FJ, San José G, Fortuño A, Fortuño MA, Díez
J.Is the balance between nitric oxide and superoxide altered in
spontaneously hypertensive rats with endothelial dysfunction?
Nephrol Dial Transplant. 2001;16 Suppl 1:2-5.
O2 and hypertension, endothelial dysfunction.
139.) Sánchez M, Galisteo M, Vera R, Villar IC, Zarzuelo A, Tamargo
J, Pérez-Vizcaíno F, Duarte J. Quercetin downregulates NADPH
oxidase, increases eNOS activity and prevents endothelial
dysfunction in spontaneously hypertensive rats. J Hypertens. 2006
Jan;24(1):75-84.
140.) Stefanovic A, Kotur-Stevuljevic J, Spasic S,
Bogavac-Stanojevic N, Bujisic N. The influence of obesity on the
oxidative stress status and the concentration of leptin in type 2
diabetes mellitus patients. Diabetes Res Clin Pract. 2008
Jan;79(1):156-63. Epub 2007 Sep 11.
141.) Dawson VL, Dawson TM.Nitric oxide in neurodegeneration.Prog
Brain Res. 1998;118:215-29.
142.) Wahl SM, McCartney-Francis N, Chan J, Dionne R, Ta L,
Orenstein JM. Nitric oxide in experimental joint inflammation.
Benefit or detriment? Cells Tissues Organs.
2003;174(1-2):26-33.143.) Bredt DS. Targeting nitric oxide to its
targets. Proc Soc Exp Biol Med. 1996 Jan;211(1):41-8.
144.) Yun HY, Dawson VL, Dawson TM. Nitric oxide in health and
disease of the nervous system. Mol Psychiatry. 1997
Jul;2(4):300-10.
145.) Berdeaux A. Nitric oxide: an ubiquitous messenger.Fundam Clin
Pharmacol. 1993;7(8):401-11.
146.) Bell D. Cellular Hypoxia & Neuro-Immune Fatigue. Wingspan
Press, July 2007.
147.) Broderick KE, Balasubramanian M, Chan A, Potluri P, Feala J,
Belke DD, McCulloch A, Sharma VS, Pilz RB, Bigby TD, Boss GR. The
Cobalamin Precursor Cobinamide Detoxifies Nitroprusside-Generated
Cyanide. Experimental Biology and Medicine 232:789-798 (2007).
148.) Broderick KE, Singh V, Zhuang S, Kambo A, Chen JC, Sharma VS,
Pilz RB, Boss GR. Nitric Oxide Scavenging by the Cobalamin
Precursor Cobinamide. J. Biol. Chem., Vol. 280, Issue 10,
8678-8685, March 11, 2005.
149.) Broderick KE, Feala J, McCulloch A, Paternostro G, Sharma VS,
Pilz RB, Boss GR. The nitric oxide scavenger cobinamide profoundly
improves survival in a Drosophila melanogaster model of bacterial
sepsis.FASEB J. 2006 Sep;20(11):1865-73.
150.) Lee ME, Wang H. Homocysteine and hypomethylation. A novel
link to vascular disease.Trends Cardiovasc Med. 1999
Jan-Feb;9(1-2):49-54.
151.) Qin J, Rosen BP, Zhang Y, Wang G, Franke S, Rensing C.Arsenic
detoxification and evolution of trimethylarsine gas by a microbial
arsenite S-adenosylmethionine methyltransferase.Proc Natl Acad Sci
U S A. 2006 Feb 14;103(7):2075-80.
152.) Dunlevy LP, Burren KA, Mills K, Chitty LS, Copp AJ, Greene
ND.Integrity of the methylation cycle is essential for mammalian
neural tube closure. Birth Defects Res. Part A Clin. Mol. Teratol.
(2006)
153.) Mason P. Folic acid - new roles for a well known vitamin. The
Pharmaceutical Journal Vol 263 No 7068 p673-677
October 23, 1999.
154.) Stempak, J. M., Sohn KyoungJin, Chiang EnPei, Shane, B., Kim
YoungIn.Cell and stage of transformation-specific effects of folate
deficiency on methionine cycle intermediates and DNA methylation in
an in vitro model. Carcinogenesis, 2005 (Vol. 26) (No. 5) 981-990.
155.) James SJ, et al Metabolic biomarkers of increased oxidative
stress and impaired methylation capacity in children with autism Am
J Clin Nutr 2004;80:1611–7.
156.) Miller A. The methionine-homocysteine cycle and its effects
on cognitive diseases. Alternative Medicine Review, Feb, 2003.
157.) Nijhout HF, Reed M, Ulrich C, Mathematical Models of
Folate-mediated One-Carbon Metabolism, in Vitamins & Hormones, Vol.
79, Folic Acid, edited by G. Litwack (Accepted, 2008), Academic
Press.
158.) Van Konynenburg R. Glutathione Depletion—Methylation Cycle
Block Hypothesis for the pathogenesis of CFS. Paper presented at
the International Association for Chronic Fatigue Syndrome, Ft.
Lauderdale, Florida, January 10-14, 2007.
159.) Yasko A. Genetic Bypass. Matrix Press. 2005.
160.) Yasko A. Nutrigenomic Testing and the Methylation Pathway.
Townsend Newsletter. 270: 69. 2006.
161.) Ganong W. F. Review of Medical Physiology, 11th Edition.
Lange Medical Publications, Los Altos California, 1983. p.235.
162.) Martin Jr. D.W., Mayes P.A., Rodwell, V.W. Harper's Review of
Biochemistry, 19th edition. Lange Medical Publications, Los Altos
California, 1983. p.312.
163.) Murphy TM, Perry AS, Lawler M.The emergence of DNA
methylation as a key modulator of aberrant cell death in prostate
cancer.Endocr Relat Cancer. 2008 Mar;15(1):11-25. 164.) Christensen
B, Ueland PM. Methionine synthase inactivation by nitrous oxide
during methionine loading of normal human fibroblasts. Homocysteine
remethylation as determinant of enzyme inactivation and
homocysteine export.J Pharmacol Exp Ther. 1993 Dec;267(3):1298-303.
165.) Christensen B, Guttormsen AB, Schneede J, Riedel B, Refsum H,
Svardal A, Ueland PM. Preoperative methionine loading enhances
restoration of the cobalamin-dependent enzyme methionine synthase
after nitrous oxide anesthesia.Anesthesiology. 1994
May;80(5):1046-56.
166.) Banerjee RV, Matthews RG.Cobalamin-dependent methionine
synthase. FASEB J. 1990 Mar;4(5):1450-9.
167.) Drummond JT, Matthews RG.Nitrous oxide degradation by
cobalamin-dependent methionine synthase: characterization of the
reactants and products in the inactivation reaction. Biochemistry.
1994 Mar 29;33(12):3732-41.
168.) Freeman AG. Hydroxocobalamin versus cyanocobalamin. J R Soc
Med. 1996 Nov;89(11):659.
169.) Linnell JC, Matthews DM. Cobalamin metabolism and its
clinical aspects. Clin Sci (Lond). 1984 Feb;66(2):113-21.
170.) Food and Nutrition Board, Institute of Medicine. Dietary
Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6,
Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline.
Washington, DC: National Academy Press; 2000.
171.) Kate E. Broderick, Prasanth Potluri, Shunhui Zhuang, Immo E.
Scheffler, Vijay S. Sharma, Renate B. Pilz, and Gerry R. Boss.
Cyanide Detoxification by the Cobalamin Precursor Cobinamide.
Experimental Biology and Medicine 231:641-649; 2006.
172.) Sharma VS, Pilz RB, Boss GR, Magde D. Reactions of nitric
oxide with vitamin B12 and its precursor, cobinamide. Biochemistry.
2003 Jul 29;42(29):8900-8.
173.) Rochelle LG, Morana SJ, Kruszyna H, Russell MA, Wilcox DE,
Smith RP. Interactions between hydroxocobalamin and nitric oxide
(NO): evidence for a redox reaction between NO and reduced
cobalamin and reversible NO binding to oxidized cobalamin. J
Pharmacol Exp Ther. 1995 Oct;275(1):48-52.
174.) Kruszyna H, Magyar JS, Rochelle LG, Russell MA, Smith RP,
Wilcox DE. Spectroscopic studies of nitric oxide (NO) interactions
with cobalamins: reaction of NO with superoxocobalamin(III) likely
accounts for cobalamin reversal of the biological effects of NO. J
Pharmacol Exp Ther. 1998 May;285(2):665-71.
175.) Brouwer M, Chamulitrat W, Ferruzzi G, Sauls DL, Weinberg JB.
Nitric oxide interactions with cobalamins: biochemical and
functional consequences. Blood. 1996 Sep 1;88(5):1857-64.
176.) Broderick KE, Singh V, Zhuang S, Kambo A, Chen JC, Sharma VS,
Pilz RB, Boss GR. Nitric oxide scavenging by the cobalamin
precursor cobinamide. J Biol Chem. 2005 Mar 11;280(10):8678-85.
Epub 2005 Jan 4.
177.) Murray, Michael. Encyclopedia of Nutritional Supplements.
Prima Publishing, 1996. Chapter 15,pg.130.
178.) Methylcobalamin. [No authors listed]. Altern Med Rev. 1998
Dec;3(6):461-3.
179.) Jepson B. Changing the Course of Autism. Sentient
Publications, 2007. Chapter 10.
180.) Kapadia CR. Vitamin B12 in health and disease: part
I--inherited disorders of function, absorption, and transport.
Gastroenterologist. 1995 Dec;3(4):329-44.
181.) Morel CF, Watkins D, Scott P, Rinaldo P, Rosenblatt DS.
Prenatal diagnosis for methylmalonic acidemia and inborn errors of
vitamin B12 metabolism and transport. Mol Genet Metab. 2005
Sep-Oct;86(1-2):160-71.
182.) Rosenblatt DS, Cooper BA, Pottier A, Lue-Shing H, Matiaszuk
N, Grauer K. Altered vitamin B12 metabolism in fibroblasts from a
patient with megaloblastic anemia and homocystinuria due to a new
defect in methionine biosynthesis. J Clin Invest. 1984
Dec;74(6):2149-56.
183.) Linnell JC, Bhatt HR.Inherited errors of cobalamin metabolism
and their management.Baillieres Clin Haematol. 1995
Sep;8(3):567-601.
184.) Watkins D, Rosenblatt DS. Genetic heterogeneity among
patients with methylcobalamin deficiency. Definition of two
complementation groups, cblE and cblG. J Clin Invest. 1988
Jun;81(6):1690-4.
185.) Suormala T, Baumgartner MR, Coelho D, Zavadakova P, Kozich V,
Koch HG, Berghaüser M, Wraith JE, Burlina A, Sewell A, Herwig J,
Fowler B. The cblD defect causes either isolated or combined
deficiency of methylcobalamin and adenosylcobalamin synthesis. J
Biol Chem. 2004 Oct 8;279(41):42742-9. Epub 2004 Aug 2.
186.) Lebionka Y., Melvidas V.I. The ability of bacterial DNA
methyltransferases to use methylcobalamine as a cofactor in DNA
methylation reactions. Biochemistry (Moscow) Supplemental Series B:
Biomedical Chemistry. 1990-7508 Issue Volume 1, Number 3 /
September, 2007.
187). Akaike A, Tamura Y, Sato Y, Yokota T. Protective effects of a
vitamin B12 analog, methylcobalamin, against glutamate cytotoxicity
in cultured cortical neurons. Eur J Pharmacol. 1993 Sep
7;241(1):1-6.
188.) Kikuchi M, Kashii S, Honda Y, Tamura Y, Kaneda K, Akaike
A.Protective effects of methylcobalamin, a vitamin B12 analog,
against glutamate-induced neurotoxicity in retinal cell culture.
Invest Ophthalmol Vis Sci. 1997 Apr;38(5):848-54.
189.) Watanabe T, et al. 1994. Ultra-high dose methylcobalamin
promotes nerve regeneration in experimental acrylamide neuropathy.
J Neurol Sci 122:140-43.
190.) Mayer G.,Kroger M., Meier-Ewert K.Effects of vitamin B12 on
performance and circadian rhythm in normal subjects.
Neuropsychopharmacology,1996, vol. 15, no5, pp. 456-464.
191.) Olle Selinus, B. J. Alloway. Essentials of Medical Geology.
Academic Press, 2005,p.519.ISBN 0126363412.
59.) The Pharmacological Basis of Therapeutics, Goodman and
Gillman, Tenth Edititon, Page-1503-1513.
192.) The Pharmacological Basis of Therapeutics, Goodman and
Gillman, Tenth Edititon, Page-1503-1513.
193.) R.S.Satoskar & S.D. Bhanderkar. Pharmacology and
Therapeutics, Revised 12th, Page No.424-425.
194.) Andrès E, Loukili NH, Noel E, Kaltenbach G, Abdelgheni MB,
Perrin AE, Noblet-Dick M, Maloisel F, Schlienger JL, Blicklé JF.
Vitamin B12 (cobalamin) deficiency in elderly patients. CMAJ. 2004
Aug 3;171(3):251-9.
195.) Rajan S, Wallace JI, Brodkin KI, Beresford SA, Allen RH,
Stabler SP. Response of elevated methylmalonic acid to three dose
levels of oral cobalamin in older adults.J Am Geriatr Soc. 2002
Nov;50(11):1789-95.
196.) Herrmann W, Obeid R, Schorr H, Geisel J.Functional vitamin
B12 deficiency and determination of holotranscobalamin in
populations at risk. Clin Chem Lab Med. 2003 Nov;41(11):1478-88.
197.) Kwok T, Tang C, Woo J, Lai WK, Law LK, Pang CP. Randomized
trial of the effect of supplementation on the cognitive function of
older people with subnormal cobalamin levels.Int J Geriatr
Psychiatry. 1998 Sep;13(9):611-6.
198.) Pimentel M, Chow EJ, Hallegua D, et al. Small intestinal
bacterial overgrowth: a possible association with fibromyalgia. J
Musculoskelet Pain 2001;9:107-113.
199.) Pimentel M, Hallegua D, Chow EJ, et al. Eradication of small
intestinal bacterial overgrowth decreases symptoms in chronic
fatigue syndrome: a double blind, randomized study.
Gastroenterology 2000;118:A414.
200.) Chandy J. Vitamin B12 Deficiency with Neuro-Psychiatric
Symptoms Serum B12 Level Below <300ng/l with or without Anaemia or
Macrocytosis: A Retrospective Study 1981- 2006 (ongoing).
Unpublished research from the Shinwell Medical Centre, Horden
Peterlee County Durham, UK. Report available on website:
http://www.b12d.org/
201.) Bradford GS and Taylor CT. Omeprazole and vitamin B12
deficiency. Annals of Pharmacotherapy 1999;33:641-3.
202.) Kasper H. Vitamin absorption in the elderly. International
Journal of Vitamin and Nutrition Research 1999;69:169-72.
203.) Howden CW. Vitamin B12 levels during prolonged treatment with
proton pump inhibitors. J Clin Gastroenterol 2000;30:29-33.
204.) Termanini B, Gibril F, Sutliff VE, Yu F, Venzon DJ, Jensen
RT. Effect of Long-Term Gastric Acid Suppressive Therapy on Serum
Vitamin B12 Levels in Patients with Zollinger-Ellison
Syndrome.American Journal of Medicine 1998; 104: 422-30.
205.) Bauman WA, Shaw S, Jayatilleke K, Spungen AM, Herbert V.
Increased intake of calcium reverses the B12 malabsorption induced
by metformin. Diabetes Care 2000;23:1227-31.
206.) Sumner AE, Chin MM, Abrahm JL, Berry GT, Gracely EJ, Allen
RH, Stabler SP. Elevated methylmalonic acid and total homocysteine
levels show high prevalence of vitamin B12 deficiency after gastric
surgery. Ann Intern Med. 1996 Mar 1;124(5):469-76.
207.) Adachi S, Kawamoto T, Otsuka M, Todoroki T, Fukao K. Enteral
vitamin B12 supplements reverse postgastrectomy B12 deficiency.Ann
Surg. 2000 Aug;232(2):199-201.
208.) Suter PM, Golner BB, Goldin BR, Morrow FD, Russel RM.
Reversal of protein-bound vitamin B12 malabsorption with
antibiotics in atrophic gastritis. Gastroenterology 1991;
101:1039-45.
209.) Súbtil JC, Betés M, Corella C, Iriarte J, Muñoz-Navas
MA.Dementia caused by bacterial overgrowth in a patient with
Billroth II gastrectomy]Rev Esp Enferm Dig. 1996 Jun;88(6):431-3.
210.) Tucker KL, Rich S, Rosenberg I, Jacques P, Dallal G, Wilson
WF, Selhub. J. Plasma vitamin B12 concentrations relate to intake
source in the Framingham Offspring Study. Am J Clin Nutr
2000;71:514-22.
211.) Louwman MW, van Dusseldorp M, van de Vijver FJ, Thomas CM,
Schneede J, Ueland PM, Refsum H, van Staveren WA. Signs of impaired
cognitive function in adolescents with marginal cobalamin status.
Am J Clin Nutr. 2000 Sep;72(3):762-9.
212.) Oh R, Brown DL. Vitamin B12 deficiency. Am Fam Physician.
2003 Mar 1;67(5):979-86.
213.) Pennypacker LC, Allen RH, Kelly JP, Matthews LM, Grigsby J,
Kaye K, Lindenbaum J, Stabler SP.High prevalence of cobalamin
deficiency in elderly outpatients.J Am Geriatr Soc. 1992
Dec;40(12):1197-204.
214.) http://www.fda.gov/fdac/departs/2007/207_upd.html#cyanide
215.) Watanabe F. Vitamin B12 sources and bioavailability. Exp Biol
Med (Maywood). 2007 Nov;232(10):1266-74.
216.) Dagnelie PC, van Staveren WA, van den Berg H. Vitamin B-12
from algae appears not to be bioavailable. Am J Clin Nutr. 1991
Mar;53(3):695-7.
217.) Lederle FA. Oral cobalamin for pernicious anemia: back from
the verge of extinction. J Am Geriatr Soc 1998;46:1125-7.
218.) Kuzminski AM, Del Giacco EJ, Allen RH, Stabler SP, Lindenbaum
J. Effective treatment of cobalamin deficiency with oral
cobalamin.Blood 1998;92: 1191-8.
219.) Lederle FA. Oral cobalamin for pernicious anemia. Medicine's
best kept secret? JAMA 1991;265:94-5.
220.) Bolaman Z, Kadikoylu G, Yukselen V, Yavasoglu I, Barutca
S,Senturk T. Oral versus intramuscular cobalamin treatment in
megaloblastic anemia: a single-center, prospective, randomized,
open-label study.Clin Ther. 2003 Dec;25(12):3124-34.
221.) National Institutes of Health Fact Sheet on vitamin B12
http://ods.od.nih.gov/factsheets/vitaminb12.asp
222.) Nilsson-Ehle H. Age-related changes in cobalamin (vitamin
B12) handling. Implications for therapy. Drugs Aging. 1998
Apr;12(4):277-92.
223.) Bishop A, Anderson JE. NO signaling in the CNS: from the
physiological to the pathological. Toxicology. 2005 Mar
15;208(2):193-205.
224.) Bucci M, Roviezzo F, Posadas I, Yu J, Parente L, Sessa WC,
Ignarro LJ, Cirino G. Endothelial nitric oxide synthase activation
is critical for vascular leakage during acute inflammation in vivo.
Proc Natl Acad Sci U S A. 2005 Jan 18;102(3):904-8. Epub 2005 Jan
7.
225.) Kaminski A, Pohl CB, Sponholz C, Ma N, Stamm C, Vollmar B,
Steinhoff G. Up-regulation of endothelial nitric oxide synthase
inhibits pulmonary leukocyte migration following lung
ischemia-reperfusion in mice. Am J Pathol. 2004 Jun;164(6):2241-9. |